Fact Sheet 2 - Transitional Cycles
Please note: The information on this page is no longer current and is only included here for historical reference.
Current information is maintained on the Price Disclosure (SPD) page.
PDF printable version of this page (PDF 330KB)
Expanded and Accelerated Price Disclosure (EAPD) commences on 1 December 2010. On that date, three of the data collection cycles under the price disclosure program prior to 1 December 2010, will be in progress (still in the data collection phase of the cycle). These cycles will become known as the Transitional Cycles.
The table below outlines the current collection period for each of these cycles and the Transitional Cycle they will become:
| Current Collection Period | Transitional Cycle |
|---|---|
| 1 January – 31 December | 1 |
| 1 May – 30 April | 2 |
| 1 September – 31 August | 3 |
This Fact Sheet will help you understand how these drugs will transition into the new processes and provisions of EAPD, including:
- which drugs will be in each Transitional Cycle;
- the timeframes for each Transitional Cycle; and
- how each Transitional Cycle merges into the Main Disclosure Cycle.
Transitional Cycle 1
Drugs in Transitional Cycle 1
The following Drugs currently disclosing for the data collection period 1 January to 31 December will form part of Transitional Cycle 1.
| Drug | Manner of Administration |
|---|---|
| Alendronic acid | Oral |
| Ceftriaxone | Injection |
| Cisplatin | Injection |
| Fluconazole | Oral |
| Fluconazole | Injection |
| Fludarabine | Injection |
| Levodopa with Carbidopa | Oral |
| Naltrexone | Oral |
| Risperidone | Oral |
| Topiramate | Oral |
| Vancomycin | Injection |
Timeframes for Transitional Cycle 1
The table below outlines the revised data collection and reporting periods for Transitional Cycle 1.
| Current Collection Period | Transitional Cycle Data Collection Start & End | Reporting Periods1 (Excluding completed Reporting periods2) | Submission Deadlines3 | Reduction Date Note this remains unchanged | Data collection flows into Main Cycle |
|---|---|---|---|---|---|
| 1 January – 31 December | 1 January 2010 – 30 September 2011 (21 months data collection) |
1 October 2010 – 31 March 2011 |
12 May 2011 | 1 April 2012 | 1 October 2011 |
| 1 April 2011 – 30 September 2011 |
11 November 2011 |
1 Including incentive data.
2 Under the price disclosure policy (prior to 1 December 2010), these drugs have completed
reporting for the period of 1 January 2010 to 30 September 2010.
3 Noting that data submitted quarterly before 01/12/10 will still be included into
WADP calculations for these transitional cycles.
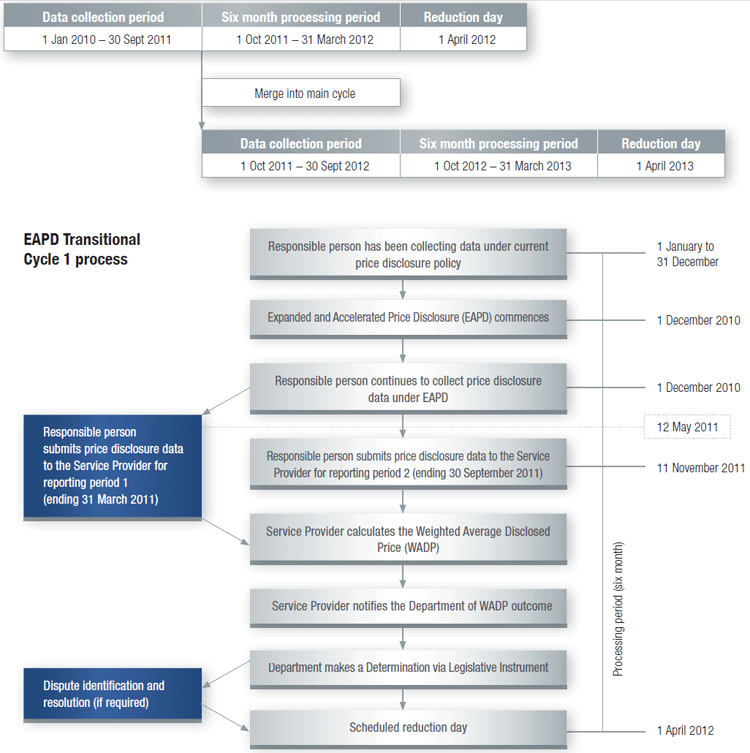
How Transitional Cycle 1 merges into Main Disclosure Cycle
Once a brand has completed the data collection period for Transitional Cycle 1 it will merge into the next available Main Disclosure Cycle, commencing on 1 October 2011, with no gap in the data collection periods (as shown in the diagram below).
Transitional Cycle 2
Drugs in Transitional Cycle 2
The following Drugs currently disclosing for the data collection period 1 May to 30 April will form part of Transitional Cycle 2.
| Drug | Manner of Administration |
|---|---|
| Carvedilol | Oral |
| Clopidogrel | Oral |
| Enalapril with hydrochlorothiazide | Oral |
| Gemcitabine | Injection |
| Irinotecan | Injection |
| Memantine | Oral |
| Paclitaxel | Injection |
| Sumatriptan | Oral |
| Vinorelbine | Injection |
Timeframes for Transitional Cycle 2
The table below outlines the revised data collection and reporting periods for Transitional Cycle 2.
| Current Collection Period | Transitional Cycle Data Collection Start & End | Reporting Periods1 (Excluding completed Reporting periods2) | Submission Deadlines3 | Reduction Date Note this remains unchanged | Data collection fl ows into Main Cycle |
|---|---|---|---|---|---|
| 1 May – 30 April |
1 May 2010 – 30 September 2011 (17 months data collection) |
1 November 2010 – 31 March 2011 |
12 May 2011 | 1 April 2012 | 1 October 2011 |
| 1 April 2011 – 30 September 2011 |
11 November 2011 |
1 Including incentive data.
2 Under the price disclosure policy (prior to 1 December 2010), these drugs have completed
reporting for the period of 1 May 2010 to 31 October 2010.
3 Noting that data submitted quarterly before 01/12/10 will still be included into
WADP calculations for these transitional cycles.
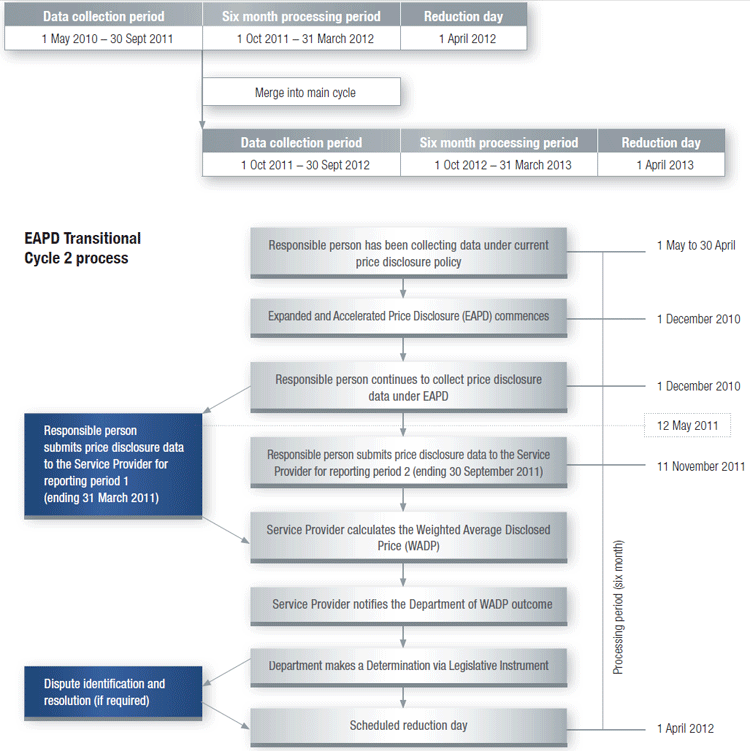
How Transitional Cycle 2 merges into Main Disclosure Cycle
Once a brand has completed the data collection period for Transitional Cycle 2 it will merge into the next available Main Disclosure Cycle, commencing on 1 October 2011, with no gap in the data collection periods (as shown in the diagram below).
Transitional Cycle 3
Drugs in Transitional Cycle 3
The following Drugs currently disclosing for the data collection period 1 September to 31 August will form part of Transitional Cycle 3.
| Drug | Manner of Administration |
|---|---|
| Alendronic acid with colecalciferol | Oral |
| Amisulpride | Oral |
| Azithromycin | Oral |
| Bicalutamide | Oral |
| Bisoprolol | Oral |
| Cabergoline | Oral |
| Cefalotin | Injection |
| Doxorubicin | Injection/Intravesical |
| Escitalopram | Oral |
| Famciclovir | Oral |
| Fosinopril with hydrochlorothiazide | Oral |
| Glucose | Injection |
| Levetiracetam | Oral |
| Meloxicam | Oral |
| Mitozantrone | Injection |
| Morphine | Oral |
| Ondansetron | Injection |
| Ondansetron | Oral |
| Oxaliplatin | Injection |
| Oxazepam | Oral |
| Oxybutynin | Oral |
| Perindopril with indapamide | Oral |
| Prochlorperazine | Oral |
| Sodium Chloride | Injection |
| Sodium Lactate Compound | Injection |
| Valproic acid | Oral |
Timeframes for Transitional Cycle 3
The table below outlines the revised data collection and reporting periods for Transitional Cycle 3.
| Current Collection Period |
Transitional Cycle Data Collection Start & End |
Reporting Periods7 | Submission Deadlines8 |
Reduction Date Note this remains unchanged |
Data collection flows into Main Cycle |
|---|---|---|---|---|---|
| 1 September – 31 August |
1 September 2010 – 31 January 2012 (13 months data collection) |
1 September 2010 – 31 March 2011 |
12 May 2011 | 1 August 2012 | 1 February 2012 |
| 1 April 2011 – 30 September 2011 |
11 November 2011 | ||||
| 1 October 2011 – 31 January 2012 |
13 March 2012 |
7 Including incentive data.
8 Noting that data submitted quarterly before 01/12/10 will still be included into
WADP calculations for these transitional cycles.
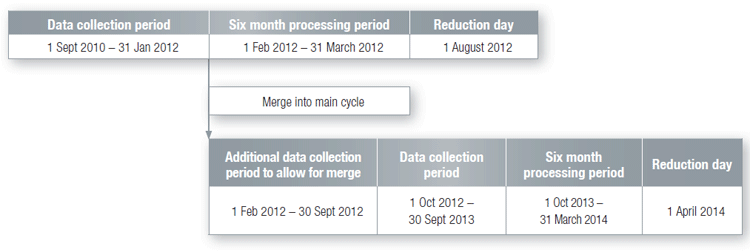
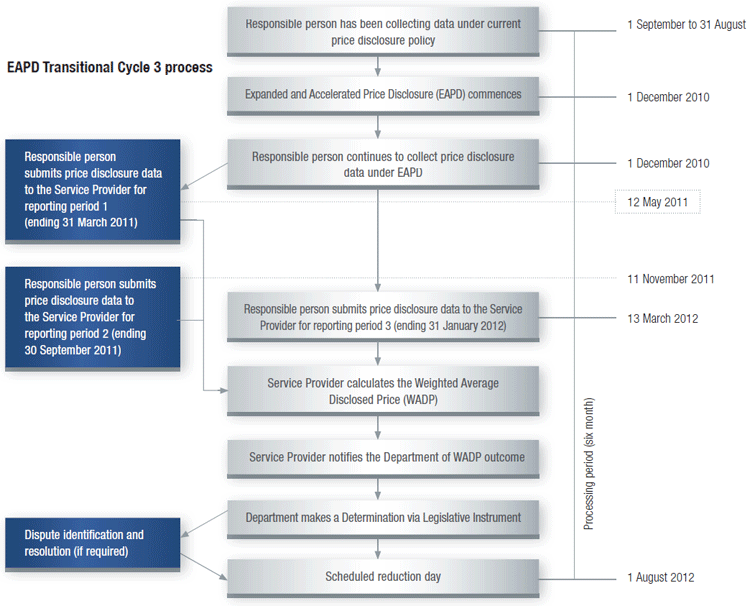
How Transitional Cycle 3 merges into Main Disclosure Cycle
Once a brand has completed the data collection period for Transitional Cycle 3 it will merge into the next available Main Disclosure Cycle, commencing on 1 October 2012, with no gap in the data collection periods (as shown in the diagram below).
EAPD Transitional Cycle 3 process
Novemeber 2012



