Symbols used in the Schedule
![]() Dentist. Indicates that the item can be prescribed by an authorised dental practitioner.
Dentist. Indicates that the item can be prescribed by an authorised dental practitioner.
![]() Medical Practitioner. Indicates that the item can be prescribed by an authorised
medical practitioner.
Medical Practitioner. Indicates that the item can be prescribed by an authorised
medical practitioner.
![]() Midwife. Indicates that the item can be prescribed by an authorised midwife.
Midwife. Indicates that the item can be prescribed by an authorised midwife.
![]() Nurse Practitioner. Indicates that the item can be prescribed by an authorised nurse
practitioner.
Nurse Practitioner. Indicates that the item can be prescribed by an authorised nurse
practitioner.
![]() Optometrist. Indicates that the item can be prescribed by an authorised optometrist.
Optometrist. Indicates that the item can be prescribed by an authorised optometrist.
RESTRICTED BENEFITS
All restricted items have separate headings for authority and non-authority items. In each case these items may be prescribed as pharmaceutical benefits only for use for one of the specified indications. Where more than one indication is specified for an Authority required or Restricted pharmaceutical benefit, each indication is separated from the preceding indication by a semi-colon and commences on the next line. In the case of Authority required (STREAMLINED) items, each indication will also include a four digit streamlined authority code. The drug may be prescribed as a pharmaceutical benefit for a patient who qualifies under any of the specified indications.
A straight line is drawn between entries for different forms and strengths of an item to indicate clearly the different restrictions which apply to these various forms and strengths.
The maximum quantity and/or number of repeats in respect of an item shown in the Schedule may be varied by the Chief Executive Medicare when approving an Authority Prescription or an Authority to Prescribe. The quantity and number of repeats shown on the authority shall be supplied. (See Explanatory Notes). Payment will be made on the basis of the price shown for that item in the Schedule.
BRAND EQUIVALENCE
'![]() ' located immediately before brand names of a particular strength of an item indicates
that the sponsors of these brands have submitted evidence that they have been demonstrated
to be bioequivalent or therapeutically equivalent, or that justification for not needing
bioequivalence or therapeutic equivalence data has been provided to and accepted by
the Therapeutic Goods Administration. It would thus be expected that these brands
may be interchanged without differences in clinical effect.
' located immediately before brand names of a particular strength of an item indicates
that the sponsors of these brands have submitted evidence that they have been demonstrated
to be bioequivalent or therapeutically equivalent, or that justification for not needing
bioequivalence or therapeutic equivalence data has been provided to and accepted by
the Therapeutic Goods Administration. It would thus be expected that these brands
may be interchanged without differences in clinical effect.
For other brands of an item, i.e., those not indicated as above, it is unknown whether or not they are equivalent. There may be several reasons for this, such as bioequivalence data not being considered necessary when the products were approved for marketing, or that advice or data have not been forthcoming from sponsors. This does not necessarily suggest a lack of safety or efficacy, but in these circumstances caution should be taken if brands are interchanged.
'![]() ' attached to brand names indicates that these brands are also equivalent, but that
it is not known if there is equivalence between brands marked '
' attached to brand names indicates that these brands are also equivalent, but that
it is not known if there is equivalence between brands marked ' 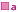 ' and brands marked '
' and brands marked ' 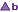 '.
'.
BRAND PREMIUM POLICY
The Brand Premium Policy was introduced on 1 December 1990 to increase price competition by allowing pharmaceutical manufacturers to set their own price on multi-branded items listed on the Pharmaceutical Benefits Scheme and to encourage the development of the generic pharmaceutical industry in Australia. The policy does this by increasing prescribers' and patients' consciousness about the price of drugs. In effect, it makes both groups question whether it is necessary for the patient to pay more for the drugs when a cheaper brand is available. The policy also allows companies to establish prices taking into account competition and consumer acceptance.
The policy operates where there is more than one brand of a particular drug available through the Pharmaceutical Benefits Scheme and where the brands are therapeutically interchangeable. Due to this, the policy mainly applies to out of patent drugs.
Basically the policy operates by:
- the Australian Government subsidising a drug to the level of the lowest priced brand (except in those instances where the lowest priced brand has, as part of its price, a therapeutic group premium);
- suppliers of other brands of that drug being able to set a price above the price charged by the supplier(s) of the lowest priced brand(s); and
- the patient paying the brand premium which is the price difference between the lowest price brand and the brand prescribed.
If a prescription is written generically or for the lowest priced brand, and the lowest priced brand is supplied, there is no brand premium payable.
'![]() ' located immediately before an amount in the premium column indicates a brand premium
which applies to that particular brand of the item.
' located immediately before an amount in the premium column indicates a brand premium
which applies to that particular brand of the item.
If a brand of a drug which is subject to a therapeutic group premium also has a brand
premium, there will be two amounts shown on separate lines in the premium column,
prefixed by '![]() ' and '
' and '![]() ' respectively.
' respectively.
If a brand of a drug which is subject to a special patient contribution also has a
brand premium, there will be two amounts shown on separate lines in the premium column,
prefixed by '![]() ' and '
' and '![]() ' respectively.
' respectively.
THERAPEUTIC GROUP PREMIUM POLICY
The Therapeutic Group Premium Policy was introduced on 1 February 1998 as an extension of the Brand Premium Policy to encourage greater competition between manufacturers of drugs and to make doctors and patients more aware of the costs of medicines.
The Therapeutic Group Premium policy applies within narrowly defined therapeutic sub-groups where the drugs concerned are of similar safety, efficacy and health outcomes.
Basically the policy operates by:
- the Australian Government subsidising drugs within a defined therapeutic sub-group to the level of the lowest priced drug in the sub-group;
- suppliers of other drugs within that sub-group being able to set prices above the price charged by the supplier(s) of the lowest priced drug; and
- the patient paying the therapeutic group premium which is the price difference between the lowest price drug and the drug prescribed.
'![]() ' located immediately before an amount in the premium column indicates a therapeutic
group premium which applies to that particular item.
' located immediately before an amount in the premium column indicates a therapeutic
group premium which applies to that particular item.
If a brand of a drug which is subject to a therapeutic group premium also has a brand
premium, there will be two amounts shown on separate lines in the premium column,
prefixed by '![]() ' and '
' and '![]() ' respectively.
' respectively.
The success of the Government in controlling prices of products supplied through the Pharmaceutical Benefits Scheme has often been criticised by the pharmaceutical industry. Under both the Brand Premium Policy and the Therapeutic Group Premium Policy, suppliers of multi-branded items and therapeutically similar drugs are able to set their own prices at a level that they think the market will bear. At the same time, the prescriber and the patient can decide whether it is necessary to pay more for a particular brand or drug when a cheaper one is available and is therapeutically interchangeable.
The brand premium or therapeutic group premium does not count toward the patient's safety net.
It should be noted that the brand premium or therapeutic group premium is not a Government charge or revenue. The premium arises from the manufacturer's price and the majority goes to the manufacturer with wholesalers and pharmacists receiving a small percentage.
SPECIAL PATIENT CONTRIBUTION POLICY
'![]() ' located immediately before an amount in the premium column indicates a special patient
contribution which applies to that particular item. See the Special Patient Contribution Premium page for more details.
' located immediately before an amount in the premium column indicates a special patient
contribution which applies to that particular item. See the Special Patient Contribution Premium page for more details.



